PERIODIC TABLE AND REACTIVITY LESSON PLAN – A COMPLETE SCIENCE LESSON USING THE 5E METHOD OF INSTRUCTION
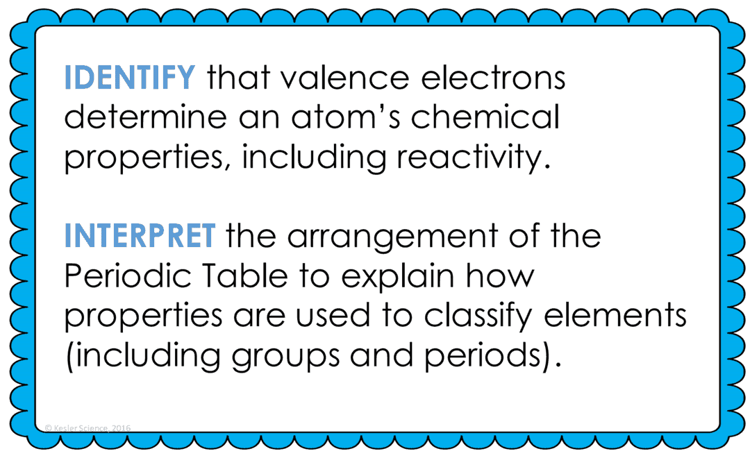
At the end of this periodic table and reactivity lesson plan, students will be able to identify that valence electrons determine an atom’s chemical properties, including reactivity and interpret the arrangement of the periodic table to explain how properties are used to classify elements (including groups and periods). Each lesson is designed using the 5E method of instruction to ensure maximum comprehension by the students.
The following post will walk you through each of the steps and activities from the periodic table and reactivity lesson plan.
ENGAGEMENT
Objective Introduction
At the beginning of the lesson, the class will do a Think-Pair-Share to discuss the objective.
Class Activity
- Give the students a periodic table.
- On the board, list everything students already know about the periodic table.
- Explain they will be looking at trends on the periodic table, one of which is the reactivity of elements. Discuss reactivity. A chemical that reacts easily with other substances is considered to be highly reactive.
- Explain that the most reactive elements are found in group 1 and they become more reactive as you go down the list. Read down the list to see if anyone recognizes these elements.
Student Activity
- Watch the YouTube video “Reaction (Explosion) of Alkali Metals with Water”
- Have them follow elements on the periodic table as the reactive properties of each element displayed.
The teacher will help to clear any misconceptions about periodic table and reactivity. Some major misconceptions are students don’t understand that all things are made up of combinations of these elements and students don’t understand that valence electrons are what determines an elements reactivity.
Estimated Class Time for the Engagement: 20-30 minutes
EXPLORATION
This student-centered station lab is set up so students can begin to explore the periodic table and reactivity. Four of the stations are considered input stations where students are learning new information about the periodic table and reactivity and four of the stations are output stations where students will be demonstrating their mastery of the input stations. Each of the stations is differentiated to challenge students using a different learning style. You can read more about how I set up the station labs here.
EXPLORE IT!
Students will be working in pairs to better understand how elements are arranged on the periodic table. Students will have cards that they will organize to give them a better visual as to what it means to be in a group or period. Students will interpret this idea as a way to show reactivity and how elements share the same properties. Students will finally take this information and apply it to an actual periodic table.
WATCH IT!
At this station, students will be watching a seven-minute video explaining the organization of the periodic table. Students will then answer questions related to the video and record their answers on their lab station sheet. For example: What does the atomic number represent on the periodic table? Which part of the atom allows one atom to react with another one? What is true about elements that are in the same column (group/family)?
RESEARCH IT!
The research station will allow students to conduct research about the construction and organization of the periodic table. Students will learn about periods, groups, and facts about elements. Students will then be directed to answer a few questions based on the research they conducted.
READ IT!
This station will provide students with a one page reading about the periodic table. In the reading, students will discover the how the periodic table is organized. Students will know what the vertical columns are called as well as the horizontal rows. There are 4 follow-up questions that the students will answer to show reading comprehension of the subject.
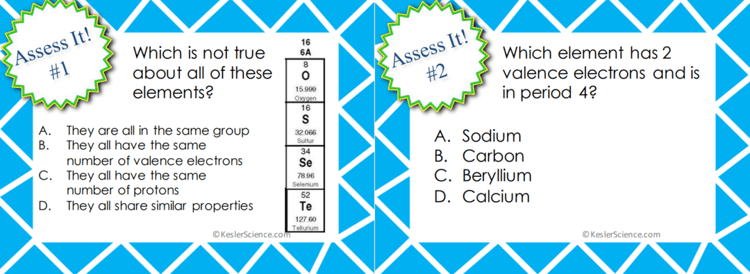
ASSESS IT!
The assess it station is where students will go to prove mastery over the concepts they learned in the lab. The questions are set up in a standardized format with multiple choice answers. Some questions include: Which is not true about all of these elements? Which element has 2 valence electrons and is in period 4? Which two elements share similar properties? Which element is the most reactive?
WRITE IT!
Students who can answer open-ended questions about the lab truly understand the concepts that are being taught. At this station, the students will be answering three task cards: Explain what a valence electron is. How are periods and groups different from each other? How can you determine if elements have the same chemical properties?
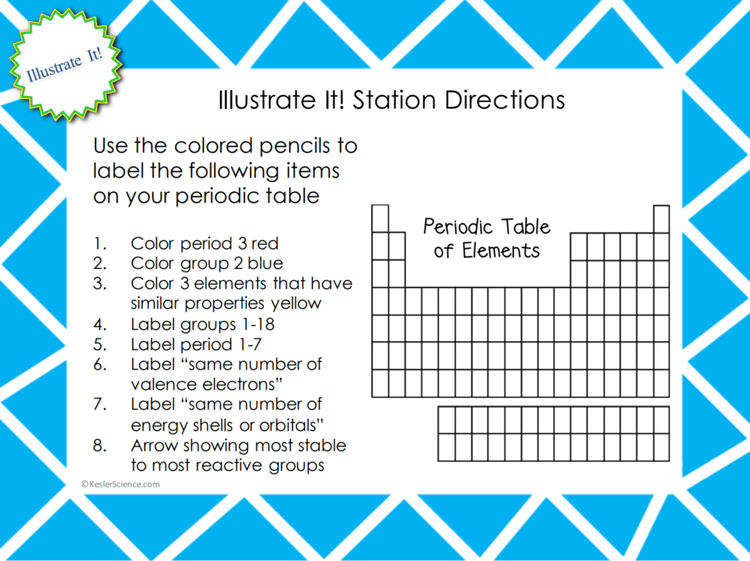
ILLUSTRATE IT!
Your visual students will love this station. Students will color in sections of a periodic table. Students will color in certain periods and groups to show that they understand how the periodic table is arranged. Students will also label certain groups, periods, and where to find elements that share the same number of valence electrons.
ORGANIZE IT!
The organize it station allows your students to place cards under other cards with the names of two certain elements. These cards will describe information pertaining to elements that can be interpreted by their location on the periodic table.
Estimated Class Time for the Exploration: 1-2, 45 minute class periods
EXPLANATION
The explanation activities will become much more engaging for the class once they have completed the exploration station lab. During the explanation piece, the teacher will be clearing up any misconceptions about the periodic table and reactivity with an interactive PowerPoint, anchor charts, and interactive notebook activities. The periodic table and reactivity lesson plan includes a PowerPoint with activities scattered throughout to keep the students engaged.

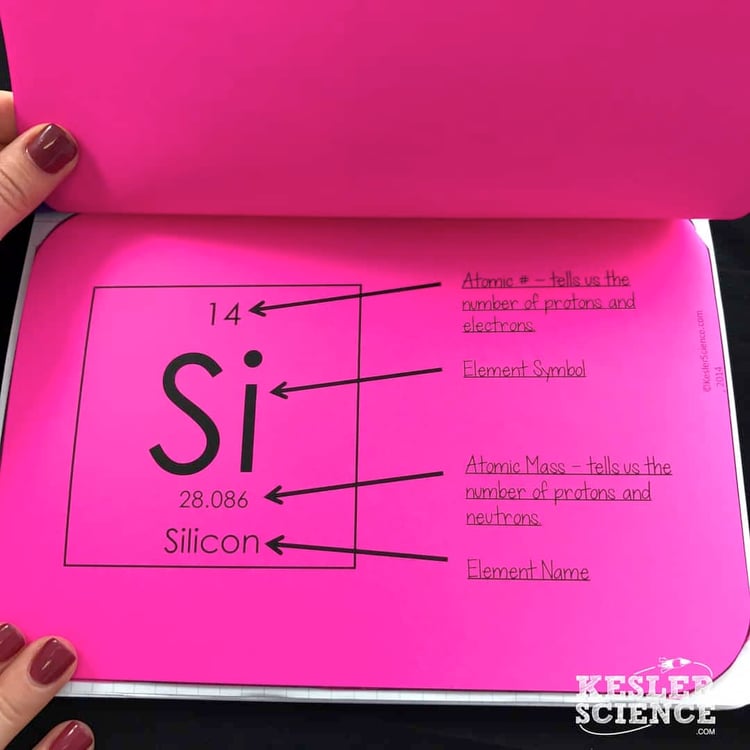
The students will also be interacting with their journals using INB templates for periodic table and reactivity. Each INB activity is designed to help students compartmentalize information for a greater understanding of the concept. The periodic table and reactivity INB templates allow students to focus their notes on learning to the difference between groups and periods, and notes on the periodic table and reactivity.
Estimated Class Time for the Exploration: 2-3, 45 minute class periods
ELABORATION
The elaboration section of the 5E method of instruction is intended to give students choice on how they can prove mastery of the concept. When students are given choice the ‘buy-in’ is much greater than when the teacher tells them the project they will have to create. The elaboration project will allow students to create a presentation to teach about the periodic table and reactivity.
Estimated Class Time for the Elaboration: 2-3, 45 minute class periods (can also be used as an at-home project)
EVALUATION
The final piece of the 5E model is to evaluate student comprehension. Included in every 5E lesson is a homework assignment, assessment, and modified assessment. Research has shown that homework needs to be meaningful and applicable to real-world activities in order to be effective. When possible, I like to give open-ended assessments to truly gauge the student’s comprehension.
Estimated Class Time for the Elaboration: 1, 45 minute class period
DOWNLOAD THE FULL LESSON NOW
The full lesson is available for download from the Kesler Science Store. Save yourself a ton of time and grab it now.
Download Over $100 in FREE Resources
For Middle School Science
Simply create a login below and gain immediate access to a selection of our Kesler Science product line worth $100 - for FREE. There's a full version of every product type! You'll also join tens of thousands of middle school science teachers who receive timely tips and strategies straight to their inbox.






