Acids and Bases Lesson Plan – A Complete Science Lesson Using the 5E Method of Instruction
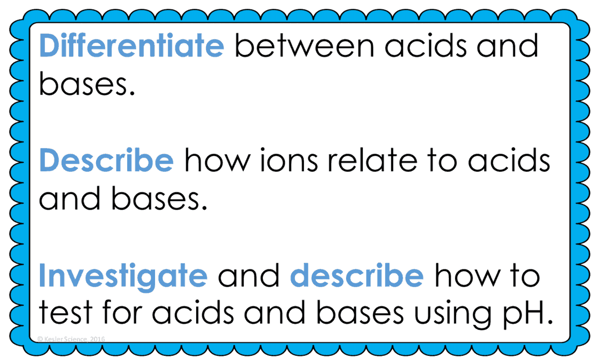
By the end of this lesson about acids and bases, students will be able to differentiate between acids and bases, describe how ions relate to acids and bases, and investigate and describe how to test for acids and bases using pH. Each of our lessons is designed using the 5E method of instruction to ensure maximum comprehension by the students. This well-thought out unit does the heavy lifting, giving teachers easy-to-implement, highly engaging lesson plans.
This blog will walk you through each of the steps and activities included in the Acids and Bases 5E Lesson Plan.
ENGAGEMENT
Objective Introduction
At the beginning of the lesson, the class will do a Think-Pair-Share to discuss the objective.
Class Activity
The teacher will show the following words on the board:
- Acid.
- Base.
- Indicator.
Demonstration:
- Set up six clear plastic cups. The first cup contains one tsp Lime-Away, the second cup one tsp vinegar, third cup water (or leave empty), fourth cup 1/4 tsp baking soda, fifth cup one tsp ammonia, sixth cup 1/8 tsp lye.
- Pour some diluted cabbage juice into each cup to about one third full.
- Swirl the cups to mix the contents.
- Different colors appear.
- Dip pH paper into each cup and compare with the pH color chart (should come with your pH paper).
Student Activity
The teacher will ask the students the following questions:
- What was the cabbage juice used for?
- How does it indicate a base?
- How does it indicate an acid?
- How does the color of the cabbage juice compare to the color of the pH paper?
- What are some household acids and bases we use every day?
- Do we eat acids and bases?
The teacher will then help to clear up any misconceptions their students may have about acids and bases. A common but major misconception, for example, is the idea that “substances containing H are acidic; substances containing OH are basic”. Many substances that contain H that are not acids and many substances that contain OH are not bases. Table sugar (sucrose), C12H22O11, contains H and OH. When dissolved in water, however, it dissolves as intact molecules and does not ionize to produce any H+ of OH- ions.
Estimated Class Time for the Engagement: 20-30 minutes
EXPLORATION
This student-centered station lab is setup so your students can begin to explore both acids and bases. With nine stations in total, you can introduce acids and bases to your middle school students in a variety of ways! Four of these stations are considered input stations where students will learn new information about acids and bases, and four of the stations are output stations where students will be demonstrating their mastery of the lesson's material. A bonus station offers challenges for your early finishers and independent learners. You can read more about how I set up the station labs here.
Watch It!
At this station, students will be watching a five and a half minute video describing acids and bases. Students will then answer some questions relating to the video and record their answers on their lab station sheet. For example, provide two examples of acids and two examples of bases from the video. Make a T-Chart to show the different characteristics of acids and bases. Describe the Arrhenius Theory. What is happening when acids and bases are ionized?
Read It!
This station will provide students with a one-page reading about acids and bases. In the reading, students will discover how almost every liquid can be classified as either an acid or base. They will learn about Svante Arrhenius and how he came up with a way to define acids and bases. Finally, students will read about the development of the pH scale. There are four follow-up questions that the students will answer to show reading comprehension of the subject.
Explore It!
Students will be working in pairs to analyze certain acids and bases. Students will have to analyze the four liquids you've set up for them. Students will use pH indicator paper to test the pH levels. Each Explore It! card will give students directions to follow and make observations and record them on their lab sheet. The final card contains three questions that have students make connections with other acids and bases that they might know.
Research It!
The research station will allow students to interact with virtual lab testing the pH levels of six different liquids. Students will grab virtual pH strips and dip them into test tubes containing stomach acid, antacid, orange juice, just to name a few. Students will then match the color on the pH strip to the chart and document the pH level for that liquid. Students will then record test results on their lab sheet.
Organize It!
The organize it station allows your students to place cards on and around a pH scale showing their understanding of how it should be labeled and where liquids end up on the scale based on their pH.
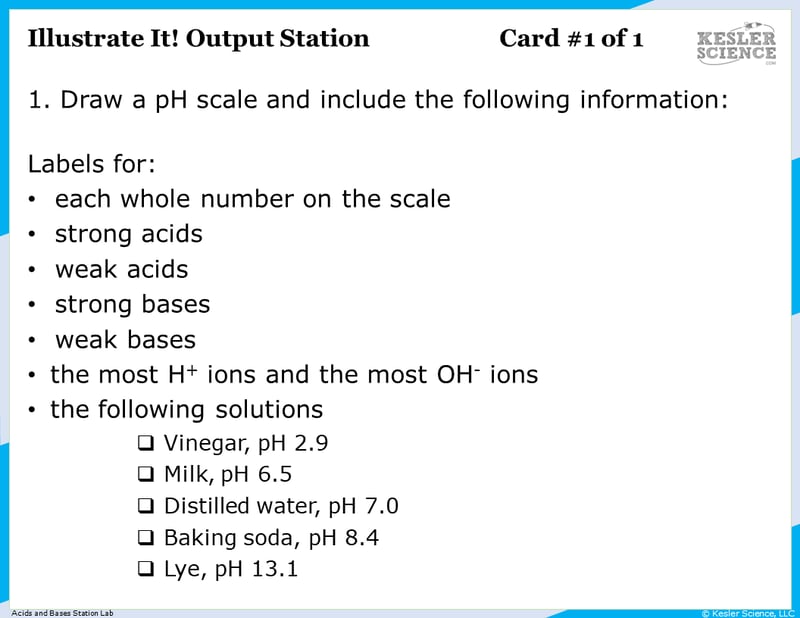
Illustrate It!
Your visual students will love this station. Students will draw a pH scale and label with numbers, strong and weak acids and bases, most H- and OH- ions, and where certain items would fall on the scale.
Write It!
Students who can answer open-ended questions about the lab truly understand the concepts that are being taught. At this station, the students will be answering three task cards to describe what the pH scale is in detail, compare and contrast acids and bases, and to describe the process of testing the pH of an unknown solution.
Assess It!
The Assess It station is where students will go to prove mastery over the concepts they learned in the lab. The questions are setup in a standardized format with multiple choice answers. Some questions will ask students: Identify the correct statement from four choices that talk about acids and bases. Based on a pH reading, which statement is true? Svante Arrhenius developed a definition of acids and bases that stated what? Which solution would be considered a weak acid?
Challenge It! - Bonus Station
Early finishers and advanced students will love the extension activities in this station. Expand their learning through crosswords, games, and mini projects.
Estimated Class Time for the Exploration: One or two 45-minute class periods
EXPLANATION
The explanation activities will become much more engaging for the class once they have completed the exploration station lab. During the explanation piece of the lesson, the teacher will be clearing up any misconceptions their students may have about acids and bases with a variety of materials. These materials include on-level and modified versions of the interactive presentation (may be used individually or projected), anchor charts, and paper or digital interactive notebook activities. If you have students that need modified notes, the 5E lessons come equipped to help give every student access to the lesson.
Interactive notebook samples: Above-left is a digital INB activity slide; above-right is an example of the paper INB activities.
The students will also be interacting with their journals using INB templates for acids and bases. Each INB activity is designed to help students compartmentalize information for a greater understanding of the concept. The acids and bases INB templates allow students to focus their notes on identifying the sections of the pH scale and where certain liquids would be placed based on their pH levels.
Estimated Class Time for the Exploration: Two or three 45-minute class periods
ELABORATION
The elaboration section of the 5E method of instruction gives students choices that allow them to prove they’ve mastered the concepts behind the lesson. When students are given a choice, they’re much more enthusiastic and invested in the project than they are when their teachers choose their projects for them. There are a total of nine choices to demonstrate understanding of acids and bases. A separate set of choices that offer more teacher support are also available for students that need them. Rubrics guide students to doing their best work and assist in grading

Estimated Class Time for the Elaboration: Two or three 45-minute class periods (can also be used as an at-home project)
EVALUATION
The final piece of the 5E model is meant to evaluate your students' comprehension of the lesson. Included in every 5E lesson is a homework assignment, assessment, and modified assessment. Research has shown that homework needs to be meaningful and applicable to real-world activities in order to be effective. When possible, I like to give open-ended assessments to truly gauge the student’s comprehension.
Estimated Class Time for the Elaboration: One 45-minute class period
DOWNLOAD THE FULL LESSON NOW
Download Over $100 in FREE Resources
For Middle School Science
Simply create a login below and gain immediate access to a selection of our Kesler Science product line worth $100 - for FREE. There's a full version of every product type! You'll also join tens of thousands of middle school science teachers who receive timely tips and strategies straight to their inbox.







